- Your cart is empty
- Continue Shopping
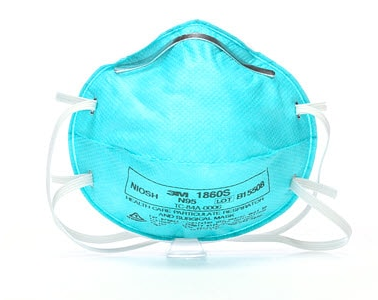
Pack of 20 – 3M™ AURA™ Health Care Particulate Respirator and Surgical Mask 1860S
$56.05
- Designed for small sized adult facial profiles; not FDA approved for use by children
- NIOSH approved N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA cleared for use as a surgical mask
- > 99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Fluid resistant according to ASTM F1862
- Not made from natural rubber latex
- Collapse resistant cup shape design
- Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
- Suggested settings and applications: Operating Rooms, Clinics, TB Wards, Patient Care, Labor and Delivery, Infection Control Practices, Laboratory, emergency or pandemic preparedness planning, stockpiling, etc.
size
Medium
Add to cart
Buy Now
Specifications
|
Aerosol Type
|
Non-Oil
|
|
Braided Comfort Strap
|
Yes
|
|
Brand
|
3M™
|
|
Cartridge or Filter Included
|
Yes
|
|
Case Quantity
|
20
|
|
Class
|
Market Leader
|
|
Exhalation Valve
|
No
|
|
Faceseal/Nosefoam
|
Nosefoam
|
|
FDA Cleared
|
Yes
|
|
Features
|
Advanced Electrostatic Media
|
|
Filter Class
|
N95
|
|
Flame Resistance (ASTM D2859-96)
|
No
|
|
Flammability Rating
|
Class 1
|
|
Fluid Resistance
|
80mm Hg
|
|
Fluid Resistant (ASTM F1862)
|
Yes
|
|
Gas & Vapor Protection Type
|
Particulates
|
|
Hazard Type
|
Mold, Silica
|
|
Individually Wrapped
|
No
|
|
Market
|
Defense, Homeland Security
|
|
M-Noseclip
|
No
|
|
Model
|
1860S
|
|
Natural Rubber Latex Components
|
No
|
|
Packaging
|
Bulk Case
|
|
Product Color
|
Teal
|
|
Product Type
|
Healthcare
|
|
Protection Level
|
N95
|
|
Purpose
|
Healthcare
|
|
Recommended Application
|
Emergency & Pandemic Preparedness
|
|
Recommended Industry
|
Health Care
|
|
Respirator Size
|
Small
|
|
Respirator Style
|
Cup
|
|
Segment
|
Personal Safety
|
|
Size
|
Small
|
|
Specifications Met
|
FDA Cleared
|
|
Strap Attachment Type
|
Braided Comfort Strap
|
This healthcare respirator is designed to help provide respiratory protection for the wearer. It meets CDC guidelines for Mycobacterium tuberculosis exposure control. As a disposable particulate respirator, it is intended to reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid resistant to splash and spatter of blood and other infectious materials.
Features
- Designed for small sized adult facial profiles; not FDA approved for use by children
- NIOSH approved N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA cleared for use as a surgical mask
- > 99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Fluid resistant according to ASTM F1862
- Not made from natural rubber latex
- Collapse resistant cup shape design
- Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
- Suggested settings and applications: Operating Rooms, Clinics, TB Wards, Patient Care, Labor and Delivery, Infection Control Practices, Laboratory, emergency or pandemic preparedness planning, stockpiling, etc.