- Your cart is empty
- Continue Shopping
Here is a look at who is eligible for expanded PCR testing, COVID-19 treatments in Ontario
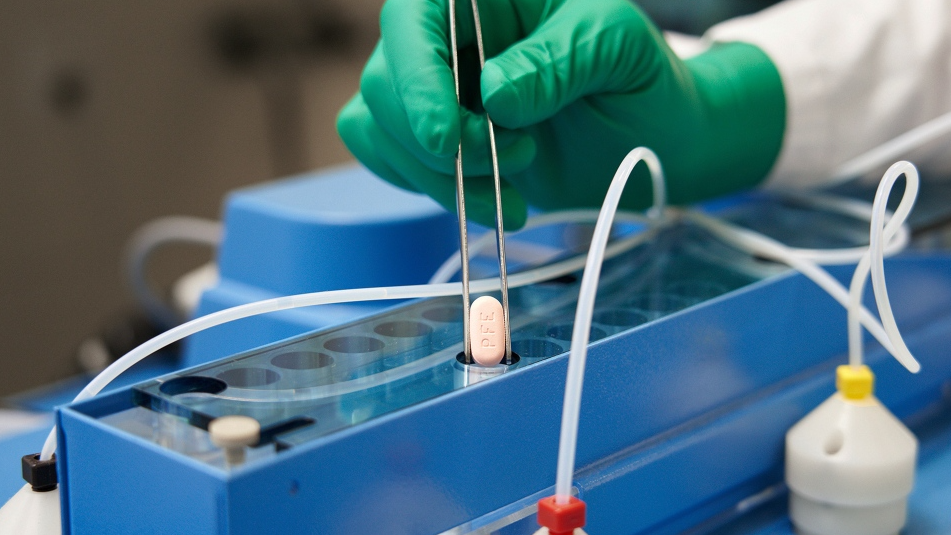
The Ontario government is making antiviral treatments and PCR testing for COVID-19 more widely available as the province grapples with the an increase in infections and hospitalizations.
The new eligibility announced on Monday includes high-risk individuals who are immunocompromised and may be at higher risk of severe side effects after contracting COVID-19.
The following groups are now eligible for PCR testing and assessment for antiviral treatments such as Paxlovid:
Individuals aged 18 and up who are immunocompromised
Individuals aged 70 and up
Individuals aged 60 and up with fewer than three vaccine doses
Individuals aged 18 and up with fewer than three vaccine doses and at least one risk condition such as a chronic medical condition.
The province is also allowing select pharmacies to dispense Paxlovid treatments with a prescription starting later this week. A full list of participating pharmacies will be available at 8 a.m. on Wednesday.
The province said antiviral treatment must begin within five days of symptoms in most cases.
As such, individuals who are part of a higher risk group and who are experiencing symptoms common for the novel coronavirus should immediately seek testing and care through their health-care provider or at a clinical assessment centre.
Patients can also use a positive rapid antigen test to be considered for antiviral treatment, officials said.
The province says they anticipate a regular supply of Paxlovid, an oral antiviral manufactured by Pfizer and approved by Health Canada earlier this year, throughout 2022.